U6 2d Motion - Review V31 Pg 4-8
Abstract
Due north half dozen-methyladenosine (mhalf-dozenA) is an abundant modification in messenger RNA and noncoding RNAs that affects RNA metabolism. Methyltransferase-like poly peptide xvi (METTL16) is a recently confirmed msixA RNA methyltransferase that methylates U6 spliceosomal RNA and interacts with the iii′-terminal RNA triple helix of MALAT1 (metastasis-associated lung adenocarcinoma transcript one). Here, nosotros nowadays two 10-ray crystal structures of the Northward-terminal methyltransferase domain (residues 1–291) of human METTL16 (METTL16_291): an apo construction at ane.9 Å resolution and a post-catalytic Southward-adenosylhomocysteine-bound complex at two.1 Å resolution. The structures revealed a highly conserved Rossmann fold that is characteristic of Class I Southward-adenosylmethionine-dependent methyltransferases and a big, positively charged groove. This groove probable represents the RNA-binding site and it includes structural elements unique to METTL16. In-depth assay of the active site led to a model of the methyl transfer reaction catalyzed by METTL16. In dissimilarity to the major thousand6A methyltransferase heterodimer METTL3/METTL14, full-length METTL16 forms a homodimer and METTL16_291 exists equally a monomer based on size-exclusion chromatography. A native gel-shift assay shows that METTL16 binds to the MALAT1 RNA triple helix, merely monomeric METTL16_291 does not. Our results provide insights into the molecular structure of METTL16, which is distinct from METTL3/METTL14.
Introduction
N six-methyladenosine (chiliad6A) is the production of a dynamic and abundant modification in eukaryotic messenger RNA (mRNA) and noncoding RNAs (ncRNA)i that affects RNA stability2, pre-mRNA processingiii, microRNA biogenesis4 and translation efficiency5. Approximately three to v thou6A sites occur in each mRNA molecule, predominantly inside 3′-untranslated regions and near terminate codons6,vii. Nearly m6A sites lie within a highly conserved RRACH (R = A or M; H = A, C or U) sequence motif1,viii and are regulated by adenosine methyltransferases ('writers'), thousand6A-binding proteins ('readers') and m6A-demethylating enzymes ('erasers')9.
Near 10006A RNA marks are catalyzed by a heterodimeric 'writer' circuitous comprised of methyltransferase-similar protein 3 and methyltransferase-like protein xiv (METTL3/METTL14), which specifically methylates adenosine within the RRACH motif with no obvious structural preferences10. Recently, methyltransferase-like protein 16 (METTL16) was confirmed to be an thousandhalf dozenA RNA methyltransferase that modifies U6 spliceosomal RNA11,12 and the MAT2A mRNA encoding S-adenosylmethionine (SAM) synthase11,13. In addition, METTL16 besides binds to ribosomal RNA, mRNA, and long ncRNAs, such every bit 10-inactive specific transcript and the 3′ triple helix of metastasis-associated lung adenocarcinoma transcript ane (MALAT1)12,fourteen. Unlike METTL3/METTL14, METTL16-dependent mviA marks do not occur within the RRACH sequence motif and they are found in introns and at intron-exon boundaries11,12. Preliminary studies advise that METTL16 uses a combination of sequence and construction to recognize its RNA substrateseleven. Interestingly, one written report has proposed that METTL16 has evolved an additional part in pre-mRNA splicing, allowing METTL16 to office as both an 1000viA 'writer' and 'reader'11. As an m6A 'author', METTL16 rapidly methylates the MAT2A mRNA in the presence of SAM, leading to intron retentivity followed past nuclear degradation11. When SAM levels are low, prolonged occupation of METTL16 on MAT2A mRNA enhances splicing of retained intron11.
METTL16 homologs are found from Escherichia coli to human and they all possess an Due north-terminal methyltransferase domain11,15,sixteen,17. As a SAM-dependent methyltransferase (SAM-MTase), METTL16 is predicted to have a highly conserved Rossmann foldeighteen. In characterized MTases, the conserved Rossmann fold is composed of alternating β strands and α helices, forming a seven-stranded β canvass that is sandwiched between clusters of α helices. In general, the SAM-bounden site is usually institute in the N-terminal segment of the β sheet, whereas the substrate-binding site lies inside the C-final segment19. Although MTases are structurally similar, they utilize a multifariousness of mechanisms to specifically recognize their targets, such as oligomerization and unique structural elements. These unique structural elements include variable loop regions within the Rossmann fold and auxiliary domains within and/or flanking the Rossmann fold20,21,22.
In this work, we nowadays the structural analysis of homo METTL16 based on ii 10-ray crystal structures of the N-last MTase domain (residues 1–291) of human METTL16 (METTL16_291): an apo form at 1.nine Å resolution and a post-catalytic complex with South-adenosylhomocysteine (SAH) at 2.1 Å resolution. We analyzed the salient MTase features of METTL16_291 based on characterized SAM-MTases; this assay highlights the unique attributes of METTL16_291 and contrasts it to the structural studies of METTL3/METTL14. Moreover, we investigated the oligomeric land of METTL16 and its power to demark to the MALAT1 RNA triple helix.
Results and Discussion
METTL16_291 is a SAM-dependent MTase with a conserved Rossmann fold
In general, m6A RNA MTases bind to SAM and RNA, transfer a methyl group from SAM to adenosine, and then the methylated RNA and SAH products dissociate from the MTase (Fig. 1A). To provide structural insights into the methyltransferase reaction catalyzed by the MTase domain of METTL16, nosotros solved X-ray crystal structures of apo and SAH-bound METTL16_291 (METTL16_291/SAH, Fig. ane and Table 1). These structures confirmed that METTL16 is a Form I SAM-MTase that uses a conserved Rossmann fold to demark the SAH product and probable SAM, the methyl-donor substrate (Figs. 1B–Eastward and S1). The conserved secondary structural elements of METTL16_291 are numbered co-ordinate to the canonical SAM-MTase fold19 (Fig. 1C,D). The conserved SAM-MTase cadre (residues 79–288) of METTL16_291 possesses a generally parallel, 7-stranded β sheet (β1–β7) organized as '3214576', with β7 existence antiparallel (Fig. 1B–D). The β canvass appears to be stabilized by a disulfide bridge between C183 and C247, thereby linking the β4 and β5 strands. This disulfide span is nowadays at approximately 40% occupancy in the apo structure and is absent in the SAH-bound complex, likely because of radiation damage and/or the presence of Tris(two-carboxyethyl)phosphine hydrochloride (TCEP) in the protein buffer. Including TCEP was necessary to prevent protein precipitation. The '3214576' β canvass is packed betwixt two clusters of α and 310 (η) helices (Fig. 1C,D). Although the MTase domain of METTL16 has a canonical Course I MTase structure, METTL16_291 has several unique structural regions: residues 1–78 that precede the Rossmann fold also every bit residues 95–97 (ηZ) and 188–222 (a putative loop containing disordered residues 188–214) within the Rossmann fold (Fig. 1C,D). Altogether, these structural elements unique to METTL16 likely contribute to its RNA substrate specificity that is distinctly different from the METTL3/METTL14 complexhalf-dozen,seven,x,11,12,22,23,24.
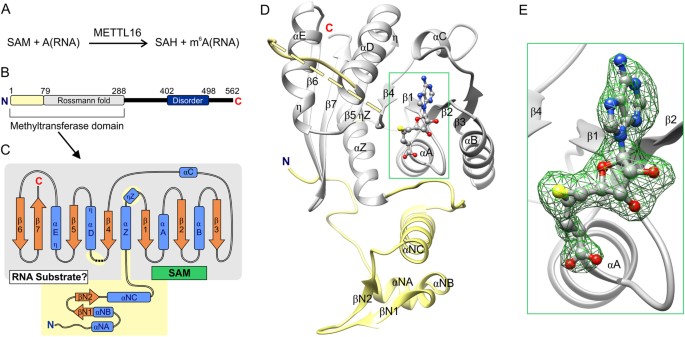
Structural overview of METTL16_291. (A) Full general scheme of methyl transfer catalyzed by METTL16. (B) Schematic diagram of the overall domain organization of man METTL16 shows the unique North-concluding region (residues 1–78, yellow) and the conserved Rossmann fold (residues 79–288, gray) in the methyltransferase domain, and a region (residues 402–498, dark blue) predicted to exist disordered by the MobiDB server41. Bluish N and red C indicate termini. (C) Schematic diagram of the secondary construction of the methyltransferase domain from METTL16_291. Blue boxes represent α and 310 (η) helices, orange arrows stand for β strands and dashed lines represent residues 188–214 that cannot be modeled. Binding sites for SAM (or SAH) and RNA are labeled within Rossmann fold. (D) Overall fold of SAH-bound METTL16_291 revealed 9 β strands, nine α helices (ii containing 3ten twist at stop of helix) and ane three10 helix. The conserved Rossmann fold of SAM-MTases is colored in gray; the structural elements colored xanthous are unique to METTL16. (E) Close-upwardly view of SAH (gray brawl-and-stick representation); green mesh represents OMIT F o -F c electron density map of SAH contoured at iv σ level.
Cofactor-binding site is defined by extensive network of noncovalent interactions
As an grand6A RNA MTase, METTL16 interacts with iii different ligands: SAM, SAH and RNA (Fig. 1A)xi,12. To report ligand bounden, we soaked METTL16_291 crystals with SAM, SAH, adenosine 5′-monophosphate (a mimic of the methyl-acceptor adenosine) and diverse RNAs but obtained merely a SAH-bound complex after soaking crystals with SAM. The presence of SAH, rather than SAM, was determined based on calculations of electron density maps (Fig. 1E) using SAM and SAH each equally models (see Methods).
Our crystal structure shows that METTL16_291 binds SAH, and probable SAM as well, inside a deep pocket of the Rossmann fold (Figs. 1D,E and 2A). Multiple hydrogen bonds ensure the proper positioning of the adenine, ribose and homocysteine moieties of SAH (Fig. 2A). The adenine moiety of SAH is recognized past the main chain of T164 in addition to a hydrophobic pocket created by the side chains of I109, V134, V160, L165 and F227 (Fig. 2). The 2′- and 3′-hydroxyl groups of the ribose are recognized via hydrogen bonding to E133, a universally conserved residue in SAM-MTases (Figs. 2A and S1)19. The sulfur atom of SAH probable hypervalently interacts25 with the courage carbonyl oxygen atoms of N184 and P186, which are part of the conserved catalytic motif (see below). R82 forms a salt bridge with the carboxyl group of SAH. Moreover, half-dozen water molecules mediate hydrogen-bonding interactions between SAH and D108, I109, T111, S114, Y117, T132, Q162 and R230 (Fig. 2A). Lastly, the nucleotide-binding site includes the conserved GXG motif (G110-T111-G112) that is characteristic of the SAM-MTase core (Fig. S1)nineteen,26. This motif shapes the cavity between adenosine and the homocysteine moieties merely only T111 interacts with SAH via a water-mediated hydrogen bond (Fig. 2A).
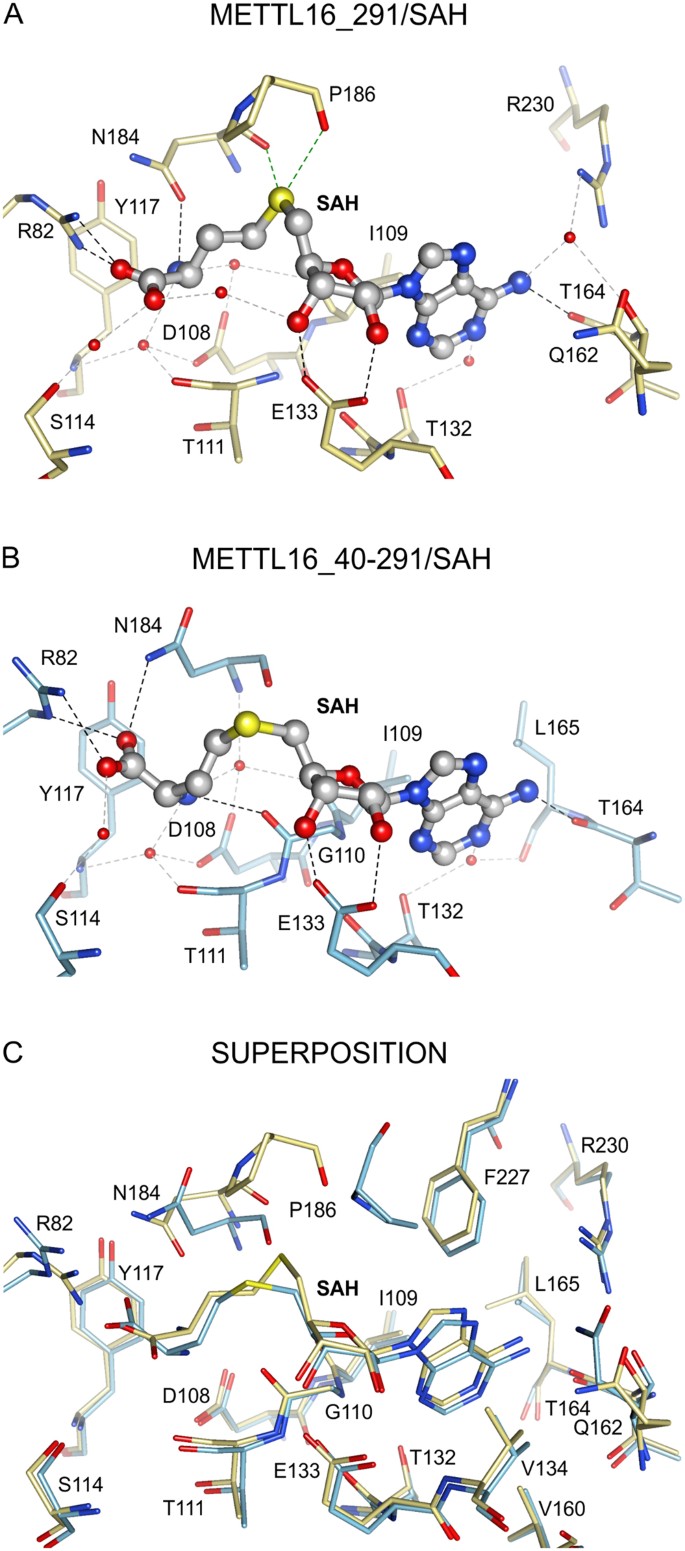
Interactions between METTL16_291 and SAH. The SAH-bounden sites are shown for (A) METTL16_291/SAH complex (yellow sticks, PDB ID: 6b92), (B) METTL16_40–291/SAH (blue sticks, PDB ID: 2h0027) and (C) superposition of METTL16_291/SAH and METTL16_40–291/SAH. Views of SAH-binding pocket are presented in the same orientation in all panels. Black, gray and green dashed lines respectively correspond noncovalent interactions via hydrogen bonds, h2o-mediated hydrogen bonds and hypervalent sulfur-oxygen bonds25 between SAH atoms and residues of METTL16. Water molecules are shown as ruby spheres. All residues forming the hydrophobic pocket (I109, V134, V160, L165 and F227) around the adenine moiety of SAH are shown together in panel C.
Next, we compared the SAH-bounden sites of our METTL16_291/SAH structure and an unpublished 10-ray crystal structure of METTL16 containing residues 40–291 (METTL16_40–291/SAH, Fig. 2B, PDB ID: 2h00, Structural Genomics Consortium27). From this comparative analysis, we discovered that most METTL16-SAH interactions overlap (Fig. 2C). However, the homocysteine moiety in METTL16_40–291, particularly the location of the sulfur atom (shifted by ii.three Å in comparison to METTL16_291), is reoriented due to the dissimilar conformations of R82 and N184 (Fig. 2C). Farther assay of SAH-bound METTL16_291 with 10-ray crystal structures of other Class I SAM-MTases (PDB ID: 1boo28, 1qan29, 1eiz30, 1hnn31, and 1khh32) indicates that the sulfur cantlet location of SAH in our circuitous is unique, which may be a crystallization antiquity resulting from an interaction between R82 and E217 of a symmetrically related molecule in the crystal lattice. Importantly, the conformation of SAH in METTL16_40–291/SAH, as preserved in other SAM-MTases, is properly oriented after a productive methyl transfer reaction. Thus, these structural snapshots suggest that METTL16_40–291/SAH represents a post-catalytic land that likely occurs before the country captured in METTL16_291/SAH.
Structural comparisons of apo and SAH-leap METTL16_291 structures revealed that the cofactor-binding site in the apo structure is occupied by four water molecules, which serve every bit placeholders for the following heteroatoms: (i) amino and carboxyl groups of the homocysteine moiety, (two) iii′-hydroxyl of the ribose moiety and (iii) N1 of adenine. Altogether, our structures indicate that METTL16 uses an extensive network of noncovalent interactions to facilitate bounden of SAH and likely SAM as well.
METTL16 has a large, positively charged groove to collaborate with RNA
To identify a putative RNA-bounden site, nosotros inspected the surface electrostatic potential of METTL16_291/SAH using the PDB2PQR server33 and institute a positively charged groove covering an expanse of ~2000 Å2, which could interact favorably with the negatively charged phosphate backbone of RNA (Fig. 3A). Residues lining the groove are highly conserved amongst Chordata METTL16 homologs (Fig. S1), thereby supporting the putative functional importance of this groove. The positively charged surface of METTL16 includes K5, K14, R41 and K47 in the unique N-terminal region; R82, K251, K252, R279 and R282 in the conserved Rossmann fold; and dipoles of the helices αNA, αNC, αZ, αηD and ηαE (Figs. 1D and 3B). The N-last residues ane–78 most probable contribute to the unique RNA substrate specificity of METTL16. Interestingly, residues K47 and R279 protrude from the surface to grade a hook-like structure broad enough (~eight Å) to arrange a phosphate group in the RNA courage (Fig. 3A).
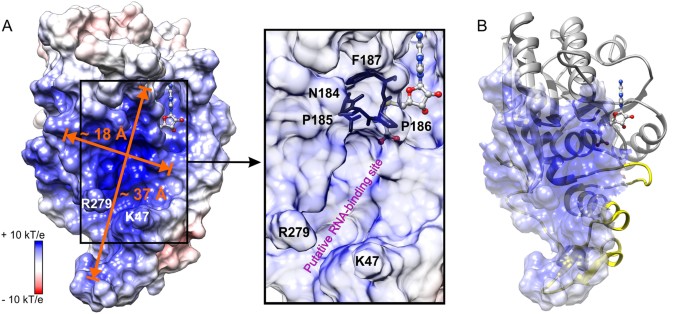
Putative RNA-bounden site in METTL16_291. (A) Surface electrostatic potential is shown for the METTL16_291/SAH complex. Groove dimensions and claw-forming residues, K47 and R279, are marked and SAH is a ball-and-stick model. The box shows a close-up view of the conserved catalytic residues, 184NPPF187, located between the putative RNA-binding site and SAH-binding pocket under semi-transparent surface. (B) Overall fold of METTL16_291 with semi-transparent representation of positively charged groove surface. Greyness and yellow colors are equally defined in Fig. one.
With our current structures of METTL16, information technology is not possible to predict how METTL16 recognizes its RNA substrates for three reasons. First, METTL16 may undergo conformational changes upon RNA binding, as observed for the 5-methylcytosine DNA methyltransferase from Haemophilus haemolyticus 34. Second, the C-terminal domain of METTL16 (residues 292–562) is missing in our structure and may also contribute to substrate recognition. 3rd, the RNA substrate specificity of METTL16 is non yet clear. The m6A sites in U6 snRNA and MAT2A mRNA, ii confirmed substrates, reside in unmarried-stranded bulges flanked by double-stranded RNA35,36. The active site of METTL16_291/SAH tin easily arrange unmarried-stranded RNA within a ~37 × 18 Å groove (Fig. 3A). Moreover, the conserved residues of METTL16 essential for catalysis, 184NPPF187, are located between the SAM/SAH-bounden site and the positively charged groove, suggesting that this region is indeed the agile site of METTL16 (Fig. 3A). Thus, the putative RNA-binding site of METTL16 is compatible with the canonical SAM-MTase architecture, whereby the RNA substrate-bounden site is located inside the C-terminal segment of the parallel β canvas and the SAM-bounden site is situated within the N-terminal segment of the parallel β sheet (Fig. 1C)19.
METTL16 uses a conserved mechanism to catalyze methyl transfer
The full general reaction machinery catalyzed by thou6A MTases has been established37,38. These enzymes contain a conserved [DNSH]PP[YFW] motif38, which corresponds to 184NPPF187 in METTL16 (Figs. 4A and S1). Contempo studies of METTL16_291 confirmed that conserved residues P185, P186 and F187 are essential for mhalf-dozenA methylation considering METTL16_291 PP185/186AA and F187G mutants failed to methylate U6 and MAT2A RNA substrates in vitro eleven. Here, we propose a model of methyl transfer from SAM to the N6 amino group of adenosine past superposing the SAH-bound METTL16_40–291 structure with an thousandsixA Deoxyribonucleic acid MTase from E. coli, EcoP15l (PDB ID: 4zcf, chain B), in complex with an extrahelical adenosine acceptor (Fig. 4B)39. The protein chains of METTL16_40–291 and EcoP15l overlap with an rmsd value of 1.8 Å across 36 Cα atoms, and the acceptor 2′-deoxyadenosine appears poised for methyl transfer in the active site of METTL16_40–291/SAH modeled with SAM. Like other 1000viA MTases21,37,38, METTL16 probable uses the Oδ atom of N184 and carbonyl oxygen of P185 to negatively polarize the N6 amino grouping of adenosine via hydrogen bonding (Fig. 4A,B). As a result, the amino group becomes primed for transfer of the methyl group from SAM via an SouthwardN2 machinery. The resulting Due north 6-methylammonium adenosine cation could exist stabilized past the same atoms that hydrogen bond to the amino group.
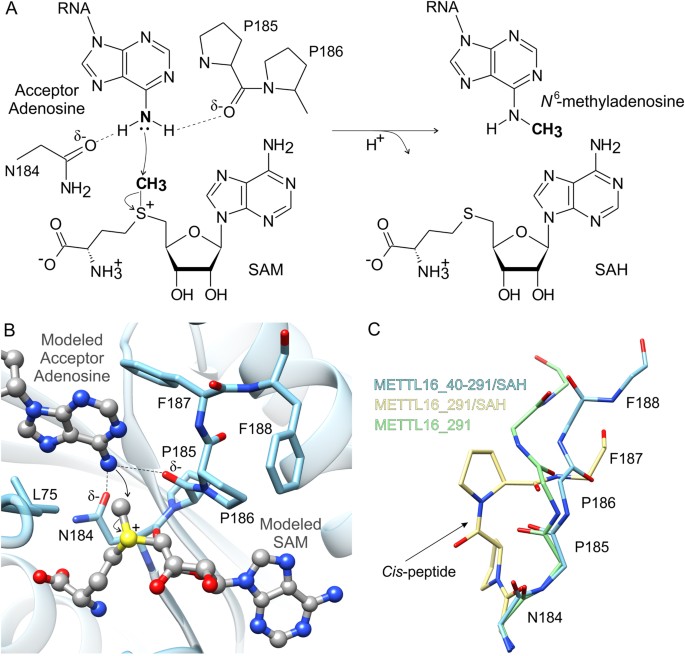
Proposed machinery of methyl transfer catalyzed by METTL16. (A) A schematic of the chemical SNorth2 reaction of methyl transfer from SAM to N six-adenosine is shown with curved arrows to bespeak motility of electron pairs. (B) To model methyl transfer, SAM (gray ball-and-stick representation) was modeled into the SAH-bounden site of METTL16_40–291 (blue) and the methyl-acceptor adenosine (greyness ball-and-stick representation) was modeled via superposition of an k6A DNA MTase, EcoP15l (PDB ID: 4zcf, chain B39), from E. coli leap to unmethylated Deoxyribonucleic acid. Residues in the 184NPPF187 motif that are disquisitional for catalysis are labeled. (C) Comparison of the apo METTL16_291 (green sticks), SAH-bound METTL16_291 (yellow sticks) and SAH-spring METTL16_40–291 (PDB ID: 2h0027, blue sticks) structures revealed different backbone conformations of the 184NPPFF188 motif; residue F188 could not be modeled in the METTL16_291/SAH structure. Side chains of P185 and P186 of METTL16_291/SAH are shown while the others have been removed for clarity. An arrow points to the cis-conformation between P185 and P186.
In general, chiliad6A MTases use the effluvious residues Y, F or Westward in the [DNSH]PP[YFW] motif to hold the extrahelical, 180°-rotated nucleobase adenine in place past π-π stacking37,38,39. In our model, the methyl-acceptor adenosine is situated in a hydrophobic environment formed by L75 and F187, but F187 is not in an optimal conformation to stack with adenosine (Fig. 4B). However, with the proper substrates, METTL16 could likely reposition F187 for optimal π-π stacking interactions considering 184NPPFF188 undergoes a conformation rearrangement upon cofactor bounden (Fig. 4C). Comparison of the apo METTL16_291, SAH-leap METTL16_291, and SAH-leap METTL16_40–291 structures revealed significantly different orientations of the 184NPPFF188 motif. Notably, apo METTL16_291 and SAH-bound METTL16_40–291 show a trans-conformation betwixt P185 and P186 whereas the SAH-jump METTL16_291 complex shows a cis-conformation (Fig. 4C). Additionally, the 184NPPF187 residues are adjacent to a disordered region spanning 189–213 in the apo structure and 188–214 in the SAH-jump complex; these residues could non be modeled due to a lack of electron density. This disordered segment is one of the elements that distinguishes the Rossmann fold of METTL16 from other SAM-MTases (Fig. 1C,D). Hence, it may play an important office in RNA substrate recognition and/or orienting molecules for catalysis.
METTL16 is a homodimeric RNA MTase
Most Class I SAM-MTases are monomeric, although some MTases grade dimers or tetramers to recognize their substrates, to assemble their agile sites or to facilitate catalysis21,37. For case, the heterodimeric METTL3/METTL14 complex forms a putative RNA-bounden site at the METTL3-METTL14 interface22,23,24 and a METTL16 ortholog in Caenorhabditis elegans exists as a homodimer40. Therefore, we used size-exclusion chromatography (SEC) to examine the oligomerization states of both human being METTL16 and METTL16_291. Our SEC data signal that METTL16 exists as a homodimer considering it elutes at a volume equivalent to a molecular weight of ~103 kDa, which is most twice the size of a unmarried METTL16 polypeptide concatenation (theoretical MW 63.7 kDa) (Fig. 5A). Furthermore, its slower migration suggests that homodimeric METTL16 has a non-globular conformation. To further investigate the oligomerization of METTL16, nosotros used SEC followed past small-angle X-ray scattering (SEC-SAXS). SEC-SAXS is a technique that uses X-ray scattering data of macromolecules in solution to determine their molecular weights, which is then used to deduce the oligomeric state of a complex eluting from the SEC column. Hither, our SEC-SAXS analysis confirmed the homodimeric country of METTL16, determining a molecular weight of 125 kDa (theoretical MW 127.4 kDa) (Fig. S2A–C). In contrast, METTL16_291, whose theoretical molecular weight is 33.3 kDa, is a monomer, eluting from SEC at a volume equivalent to ~31 kDa (Fig. 5A). These results propose that the C-terminal domain of human METTL16, which contains an evolutionarily variable region (residues 402–498) predicted to be disordered past MobiDB41, is required for oligomerization (Figs. 1B and S1). This finding is consequent with the oligomerization studies of the METTL16 ortholog in C. elegans xl. Notably, homodimerization of METTL16 does not appear to be required for RNA binding and MTase activity because METTL16_291 methylates U6 and MAT2A RNA substrates in vitro 11.
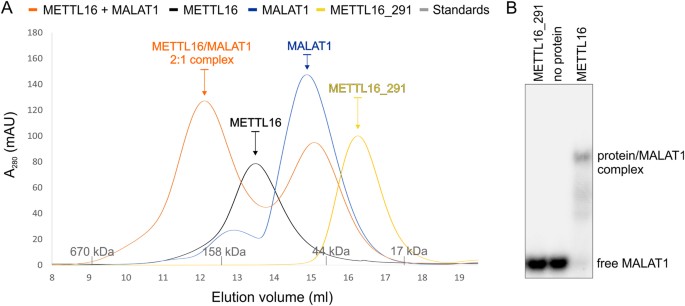
Analysis of METTL16-MALAT1 RNA triple helix interaction. (A) SEC revealed that METTL16 exists equally a homodimer (SEC MW: ~103 kDa, black) while METTL16_291 is a monomer (SEC MW: ~31 kDa, yellow). SEC of the METTL16/MALAT1 RNA triple helix complex (SEC MW: ~188 kDa, orangish) indicates a stoichiometry of 2:one and likewise has a peak that elutes similar to the MALAT1 RNA triple helix (SEC MW: ~56 kDa, blueish). Molecular weights of protein standards are shown every bit grayness tick marks on x-centrality. (B) Gel-shift assay showing that purified METTL16 (0.5 μM dimer) interacts with v′-[32P]-labeled MALAT1 RNA triple helix (2 nM), whereas METTL16_291 (1 μM) does not. This gel image was cropped; the full-length gel image is shown in Fig. S3.
Recently, METTL16 was shown to interact with the RNA triple helix at the three′ end of MALAT114. Therefore, we used a native electrophoretic mobility shift analysis (EMSA) to determine if METTL16 and METTL16_291 can interact with the MALAT1 RNA triple helix in vitro. Our results testify that monomeric METTL16_291 (1 μM) does not bind to the MALAT1 RNA triple helix but METTL16 (0.5 µM dimer) does bind (Fig. 5B). Next, we used SEC and SEC-SAXS to examine the stoichiometry of the METTL16/MALAT1 RNA triple helix circuitous. SEC data showed that MALAT1 elutes at a volume equivalent to a molecular weight of ~56 kDa (Fig. 5A) whereas SEC-SAXS determined a molecular weight of 36 kDa (theoretical MW 30.2 kDa) (Fig. S2D–F). The apparent college molecular weight of MALAT1 RNA is consistent with previous SEC studies showing that RNAs carry every bit globular proteins upwards to five fold larger than their theoretical molecular weights42. When METTL16 is mixed with the MALAT1 RNA triple helix at a 1:1 molar ratio, the SEC profile showed two peaks: one peak respective to the METTL16/MALAT1 RNA circuitous at ~188 kDa and i peak respective to unbound RNA at ~51 kDa (Fig. 5A). SEC-SAXS was used to examine the METTL16/MALAT1 RNA peak, and these results revealed a molecular weight of 155 kDa (theoretical MW 157 kDa), indicating that the METTL16 homodimer interacts with one molecule of MALAT1 RNA (Fig. S2G–I). Thus, these EMSA, SEC and SEC-SAXS results propose that METTL16 may utilize different mechanisms to interact with unlike RNAs considering just dimeric METTL16 interacts with the MALAT1 RNA triple helix but monomeric METTL16_291 binds and methylates both U6 and MAT2A RNAseleven.
Structural comparison of METTL16_291 and METTL3/METTL14
METTL16 is the second catalytically active mviA mRNA MTase to be validated in humansxi. The only other mhalf dozenA mRNA MTase currently known is the heterodimeric METTL3/METTL14 complex, whereby METTL3 has MTase activity and METTL14 functions as a scaffold to bind RNA22,23,24. Therefore, nosotros superposed the 10-ray crystal structures of human METTL3 (PDB ID: 5il2, residues 369–570) and human METTL14 (PDB ID: 5il2, residues 117–420) in complex with SAH22 onto SAH-bound METTL16_291. This comparative structural analysis revealed that METTL16_291 is like to merely METTL3 (rmsd 2.i Å across 40 Cα atom pairs) and not METTL14 (Fig. half-dozen). In METTL3, its conserved Rossmann fold includes 4 α helices, three iii10 helices and an eight-stranded parallel β canvas organized as '18723546', with the β5 strand being antiparallel22 (Fig. 6A,B). This β-sheet organization is distinctly different from that of METTL16_291 ('3214576' in Fig. 6B), which explains the lack of sequence similarity between METTL16 and METTL3. Withal, the 3D spatial positioning of β strands and α helices within the Rossmann fold is similar except for the unique β6 strand and α4 helix of METTL3 and the unique αB helix of METTL16 (Fig. 6B). The SAH-binding sites in METTL3 and METTL16_291 are conserved in the Rossmann fold, although balance identities are dissimilar. Nonetheless, METTL16 and METTL3 form noncovalent interactions with the backbone atoms of homocysteine and N6, N1, 2′-hydroxyl and 3′-hydroxyl groups of adenosine in SAH.
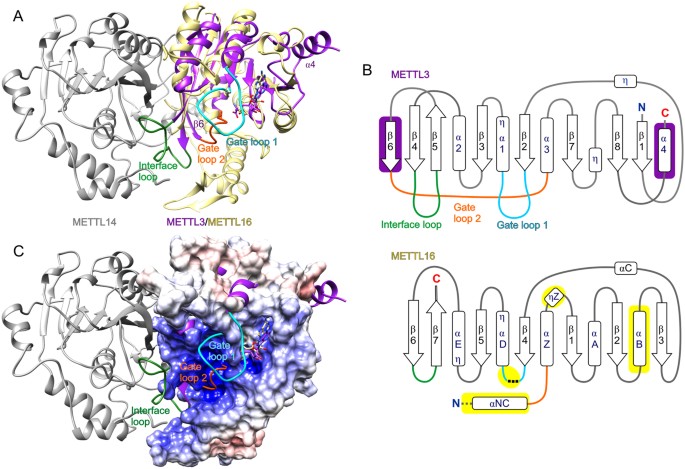
Structural comparing of METTL16_291 and METTL3/METTL14 complex. (A) METTL16_291/SAH (yellow drawing) superposed on the human METTL3 (purple cartoon)/METTL14 (gray cartoon)/SAH complex (PDB ID: 5il222). Gate loops and the interface loop of METTL3 are colored as follows: gate loop 1 is cyan, gate loop 2 is orange and interface loop is green. The unique β6 strand and α4 structures of METTL3 are labeled. (B) Schematic representation of Rossmann fold from METTL3 (residues 369–570) and METTL16 (residues 49–291). Arrows represent β-strands and boxes represent α and 310 (η) helices. Elements unique to METTL3 are shown on royal groundwork, elements unique to METTL16 are shown on yellow background and elements of METTL16 analogous to the gate loops and interface loop of METTL3 are colored as defined in console A. Black dashed lines stand for residues 188–214 that cannot be modeled and grayness dashed lines correspond N-terminal structural elements of METTL16 omitted for clarity. Helices of METTL3 are numbered as previously described22. (C) Surface electrostatic potential of METTL16_291/SAH is superposed on the two gate loops and interface loop of the METTL3/METTL14 (purple cartoon/gray cartoon) complex (PDB ID: 5il222).
The structural ground of RNA recognition by METTL16 and METTL3/METTL14 remains unclear. However, it has been proposed that the gate loops of METTL3/METTL14 play a role in RNA substrate recognition22. These gate loops map to the same face of the protein construction as the positively charged groove of METTL16_291 (Fig. 6C). More precisely, gate loop i of METTL3 (residues 395–410) corresponds to residues 184–222 in METTL16, a predicted loop that includes disordered residues 188–214 (Fig. 6B). These regions include the conserved catalytic residues: 395DPPW398 in METTL3 and 184NPPF187 in METTL16. The structural counterpart of gate loop 2 in METTL3 (residues 507–515) is the loop betwixt αNC and αZ as well every bit N-concluding office of αZ in METTL16_291 (Figs. 1C,D and 6B). Both of these regions contribute to the positively charged groove (Fig. 6C). Interestingly, the long interface loop of METTL3 (residues 462–479) that interacts with METTL14 corresponds to the short β hairpin betwixt strands β6 and β7, which contains R279 involved in the K47-R279 claw-like structure, in METTL16 (Figs. 3A and 6B). Thus, this comparative analysis shows that structural elements unique to METTL3 and METTL16 are located primarily in the RNA-binding site, providing a structural basis for why METTL3/METTL14 and METTL16 recognize different RNA substrates6,vii,10,11,12,22.
Role of METTL16 as an m6A RNA MTase
mRNA modifications, especially one thousand6A, accept been intensely studied in the past few years. Much of the work has focused on METTL3/METTL14, although information technology is now clear that METTL16 too plays a key role in m6A biology11,12,13. Recent studies suggest that METTL16 does not follow trends more often than not associated with yardsixA: (i) RNA targets do not have a RRACH motif and (two) bulk of METTL16-dependent thou6A marks are found in introns1,8,11,12,43. These findings might not be that surprising because METTL16 knockdown accounts for a ~20% loss of the mhalf-dozenA methylome in 293A-TOA cells11. Notwithstanding, METTL16, which is distributed throughout the nucleus of HeLa cells, interacts with a broad range of RNAs, including long ncRNA, mRNA, miRNA, rRNA, snRNA and snoRNA11,12,14. Validated mhalf dozenA targets include U6 snRNA, whose methyl mark at A43 is apparently essential for spliceosome activity, and MAT2A mRNA; both RNAs are methylated by METTL16 in a UACmsix_AGA sequence11,12,13. METTL16 also interacts with the MALAT1 RNA triple helix, which is 13 base pairs from a weak m6A indicate most A8290 in a ACAm6_ACA sequencexiv,44. Thus, it will be interesting to decide if the MALAT1 RNA triple helix itself or side by side regions are substrates of METTL16 considering MALAT1 is a cancer-promoting long ncRNA45. Furthermore, our studies bear witness that full-length METTL16 is required to bind to the MALAT1 RNA triple helix, suggesting that the C-terminal domain expands the RNA interactome of METTL16. Two regions of the C-terminal domain of METTL16 (residues 289–399 and 514–562) are conserved in vertebrates11 while the N-last MTase domain is conserved from East. coli through human being (Figs. 1B and S1). METTL16_291 shares 31% identity and 50% similarity to a METTL16 homolog in E. coli: RlmF (or ybiN), which methylates A1618 in 23 South rRNA15. Both METTL16 and RlmF can install chiliad6A marks within the ACm6_AGR sequencexi,thirteen,xv. Man METTL16 interacts with eighteen South and 28 Southward rRNAs, which contain m6A marks at A1832 and A4220, respectively12,14,46,47,48. These marks occur within a GUAm6_ACR sequence, which would appear to be a suboptimal substrate for METTL3/METTL14 and maybe METTL16. Interestingly, the Amsix_ACR motif found in rRNA besides appears at A8290 in MALAT1 RNA. Some other factor that may influence RNA substrate recognition and MTase action is protein cofactors. Thus far, La protein, La-related protein 7, and methylphosphate capping enzyme take been identified as binding partners of METTL16 in a U6 snRNA-dependent manner12. Thus, more cellular, biochemical, and structural studies of METTL16 are necessary to elucidate its part at the molecular level.
Conclusions
Our work expands the structural knowledge of human thousandhalf-dozenA RNA MTases by analyzing the ligand-binding sites and proposing a structural model of methyl transfer catalyzed by METTL16. Interestingly, METTL16 has an extensive, positively charged groove to demark RNA and this groove is largely comprised of structural elements unique to METTL16, suggesting this surface area may contribute to the RNA substrate specificity of METTL16 that is distinctly different from METTL3/METTL14. Moreover, our SEC and SEC-SAXS information show that METTL16 is a homodimer; yet, the Northward-terminal domain of METTL16 is a monomer, indicating that the C-terminal domain facilitates protein dimerization. Homodimeric METTL16 is required to bind to the MALAT1 RNA triple helix while monomeric METTL16_291 is sufficient to methylate U6 and MAT2A RNAs11. Hopefully, future studies will reveal the biochemical and structural ground of the unique RNA substrate specificity of METTL16 and its roles every bit an m6A 'writer' and 'reader'.
Methods
Cloning, overexpression and purification of METTL16_291 and METTL16
The sequences encoding human METTL16_291 (residues one–291) and METTL16 (residues 1–562) [UniProt ID: Q86W50] were amplified by PCR using a pcDNA3-METTL16 plasmid and the following primers: Frontward: 5′-TACTTCCAATCCAATGCCATGGCTCTGAGTAAATCAATGCATGCAA-3′ and Contrary: v′-TTATCCACTTCCAATGTTACTAATCATAAAAACTCCAAGCTAAGGCC-3′ for METTL16_291; Forward: 5′-TACTTCCAATCCAATGCCATGGCTCTGAGTAAATCAATGCATGCAA-three′ and Reverse: 5′-TTATCCACTTCCAATGTTACTAGTTAACTGCAACAAGCCTGAAAATTTG-3′ for METTL16. Then, the Deoxyribonucleic acid insert was incorporated into the pMCSG68 vector (Midwest Center for Structural Genomics) using a ligase-independent cloning method49. The expressed METTL16_291 and METTL16 proteins have an N-final Hishalf-dozen-tag followed by a Tobacco Etch Virus (TEV) protease cleavage site and a Ser-Asn-Ala linker, which is encoded in the pMCSG68 vector. The sequence of the factor was confirmed by DNA sequencing (University of Notre Dame Genomics Facility).
Protein overexpression was carried out in BL21 Gold E. coli (Agilent Technologies) cells grown in 2 L LB media supplemented with 150 μg/ml ampicillin. The leaner were cultured with shaking at 190 RPM at 37 °C until the OD600 exceeded 1.0 (~4 h). After, cultures were chilled to eighteen °C, and protein expression was induced by the add-on of 0.5 mM isopropyl-D-thiogalactopyranoside for 18 h. Cells were harvested by centrifugation at 5,000 x g for xv min at 4 °C, resuspended in ice-cold bounden buffer [fifty mM HEPES-NaOH pH 7.5 at RT, 500 mM NaCl, 20 mM imidazole and 1 mM tris(two-carboxyethyl)phosphine (TCEP)] and stored at −80 °C.
Thawed cells were disrupted by sonication (iv min total of probe working time), using 4-s bursts with 26-s intervals for cooling in an water ice/water bathroom. Lysate was cleared by centrifugation at 26,000 x g for xl min at 4 °C. The supernatant was transferred to a 50-ml column packed with 3 ml of pre-done HisTrap HP resin (GE Healthcare) and incubated for 5 min at 4 °C. The column was connected to a Vac-Man Laboratory Vacuum Manifold (Promega) to wash the poly peptide-bound resin half dozen times with 50 ml binding buffer for each wash. The His6-tagged protein was eluted by gravity with 20 ml elution buffer (l mM HEPES-NaOH pH seven.v at RT, 500 mM NaCl, 400 mM imidazole and one mM TCEP). The imidazole concentration was lowered to 20 mM and the His6-tag was cleaved with TEV protease (last concentration 0.1 mg/mL) during dialysis for 18 h at 4 °C. The sample was reapplied onto a HisTrap HP resin to remove both the broken Hisvi-tag and the Hishalf dozen-tagged TEV protease. Period-through was collected and concentrated to ii.0 ml using Amicon ultra centrifugal filters (30-kDa cutoff, Millipore). The poly peptide was further purified by SEC (HiLoad 16/600 Superdex 200 pg, GE Healthcare) using size exclusion buffer (SEB) containing 25 mM HEPES pH 7.5 at RT, 100 mM KCl, 50 mM NaCl and 1 mM TCEP. The peak fractions of METTL16_291 and METTL16 were pooled and full-bodied to 27 mg/ml and 10 mg/ml, respectively. A280 values were measured and molarity calculated using an extinction coefficient of 43890 and 63370 L/(mole·cm) for METTL16_291 and METTL16, respectively.
Crystallization and diffraction data collection
Crystals grew at 19 °C using the hanging-drop method. The crystallization driblet independent 4 µl METTL16_291 (27 mg/ml) and two µl of reservoir solution, which independent ane.3 1000 K2HPOfour, 45 mM NaH2PO4, pH 8.five. Crystals measuring approximately 0.3 × 0.3 × 0.2 mm appeared after iii days. To obtain the SAH-bound METTL16_291 circuitous, 0.2 µl of 200 mM SAM solution (Sigma-Aldrich, buffered in 50 mM HEPES pH seven.5) was added to the drib with mature crystals. Crystals were cryoprotected by soaking in crystallization solution supplemented with 25% ethylene glycol, vitrified in liquid nitrogen and stored until data collection. Diffraction data were collected under cryocooled conditions (100 K) at the Avant-garde Photon Source, Argonne National Laboratory, on beamline 22-ID. XDS50 package was used for information reduction. The statistics of the information collection and processing are summarized in Table 1.
Decision and refinement of the crystal structures
The crystal structures of METTL16_291 were solved past molecular replacement using Phaser51 and the X-ray crystal structure of homo methyltransferase 10 domain containing protein (94% identity, PDB ID: 2h0027). ARP/wARP52 was used to build the initial model, which afterwards was placed inside the unit prison cell with the ACHESYM server53. Transmission plumbing fixtures in the electron density maps was completed in COOT54 with iterative rounds of model refinement in Refmac55. Ten and four TLS56,57 groups were added for apo and SAH-jump METTL16_291, respectively, as recommended by the TLSMD server58. The refinement statistics are listed in Table 1. Our final models include residues three–291 (missing 189–213) in apo METTL16_291 and 5–291 (missing 188–214) for SAH-bound METTL16_291. The presence of SAH, rather than SAM, was determined based on the electron density maps calculated for refined models with either of the two ligands. No peak of positive density appeared at the position corresponding to the methyl group when SAH was placed in the model, whereas for SAM, the methyl group lied in a acme of negative density. A sodium cation in the apo structure was modeled on the basis of octahedral coordination sphere (with four out of half-dozen electron pair donors) and the interatomic distances that refined to values below 2.4 Å. 3 and four ethylene glycol molecules were modeled in the apo and SAH-bound METTL16_291 structures, respectively.
Belittling SEC
The 94-nt MALAT1 RNA triple helix 5′-GGAAGGUUUUUCUUUUCCUGAGAAAACAACACGUAUUGUUUUCUCAGGUUUUGCUUUUUGGCCUUUUUCUAGCUUAAAAAAAAAAAAAGCAAAA-3′ was generated using the pHDV-MALAT1 ENE + A WT plasmid equally described previouslyfourteen,59. All samples (METTL16_291, METTL16, MALAT1 RNA triple helix and METTL16/MALAT1 RNA triple helix) were diluted to ~21 µM in 100 µl full volume of SEB buffer supplemented with 1 mM MgCl2. RNA was denaturated at 95 °C for ane min, snap-cooled on ice for five min and incubated at room temperature for ane h. METTL16 and the MALAT1 RNA triple helix were mixed at a one:1 ratio of 21 μM:21 μM and incubated at room temperature for 45 min prior to injection. Samples were injected onto a Superdex 200 10/300 GL column (GE Healthcare) pre-equilibrated with SEB buffer supplemented with i mM MgClii and resolved at a menses rate of 0.vi ml/min. Thyroglobulin (670 kDa), gamma-globulin (158 kDa), ovalbumin (44 kDa) and myoglobin (17 kDa)) were used every bit standards (Gel Filtration Standard, BioRad). Molecular weights of METTL16_291, METTL16, MALAT1 RNA triple helix and METTL16/MALAT1 RNA triple helix circuitous were calculated using a standard curve.
Small-angle 10-ray handful (SAXS) measurements
Solution SAXS data were collected at the Advanced Photon Source BioCat, 18-ID beamline in the SEC-SAXS pipelinethreescore. Samples pre-equilibrated in SEB buffer supplemented with ane mM MgClii were injected onto the SEC column at the post-obit concentrations: xxx μM METTL16, 25 μM MALAT1 RNA and METTL16 + MALAT1 RNA complex at a 2:1 ratio of 30 μM:15 μM. Data were recorded at room temperature on a PILATUS 3 ane M detector with 0.5-s exposures every 3 s at one.0 Å wavelength. Sample jail cell-to-detector distance was 3.v m, and the data range was 0.0163 Å−1 to 0.3666 Å−ane (q = 4π sin θ/λ, where 2θ is the scattering angle, and λ is the Ten-ray wavelength). Baseline scattering was averaged from data points measured before and later the protein peak, whereas sample frames corresponding to the elution peak were averaged to maximize the point-to-noise ratio. Data were candy using the BioCAT beamline pipeline, which is based on ATSAS package61. Guinier assay was performed in PRIMUS 62 and GNOM 63 for calculation of the radius of gyration (Rg) and the pair distribution function, P(r). Molecular masses are based on the calculation of low-resolution ab initio models with the use of DAMMIF 64, DAMAVER 65, DAMMIN 66, and DAMFILT.
Electrophoretic mobility shift assay (EMSA)
The MALAT1 RNA triple helix was 5′-end radiolabeled with [γ-32P]ATP equally described previously14. Immediately before incubating RNA with protein, RNA was denaturated at 95 °C for 1 min, snap-cooled on ice for 5 min, and incubated at room temperature for 1 h. Then, two nM 5′-[32P]-labeled MALAT1 RNA was mixed with 1 μM METTL16_291 or METTL16 in binding buffer (25 mM Tris pH 7.five, 25 mM NaCl, 150 mM KCl, 1 mM MgClii, ane mM DTT, 0.v mg/ml tRNA, 1U/μl RNase Inhibitor and 8% glycerol) at room temperature for 45 min. The reactions were resolved using five–6% native polyacrylamide gel (nineteen:ane acrylamide:bisacrylamide, one × Tris-Borate buffer (TB), 1 mM MgCl2) and running buffer (0.75 × TB, 1 mM MgCl2). Electrophoresis was run under an electrical field of 8 V cm−1 for two.5 h. Gels were visualized on phosphor screen and scanned using an Amersham Typhoon IP Biomolecular Imager (GE Healthcare).
Other software used
Disordered region of METTL16 was calculated past MobiDB server41. Molecular illustrations were created using UCSF Chimera67, which also calculated rmsd values across pairs of Cα atoms inside 3 Å radius for all superposition analyses. Surface electrostatic potential was calculated using the PDB2PQR server33. The amino acrid sequences of METTL16 from 394 chordates were aligned using ClustalX268 and conservation calculated using the ConSurf server69.
Accession numbers
Coordinates and construction factors were deposited in the Protein Data Bank (PDB): apo METTL16_291, PDB ID: 6b91; METTL16_291/SAH complex, PDB ID: 6b92.
References
-
Pan, T. N 6-methyl-adenosine modification in messenger and long non-coding RNA. Trends Biochem Sci 38, 204–209 (2013).
-
Wang, X. et al. Northward vi-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
-
Liu, N. et al. N half-dozen-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564 (2015).
-
Alarcon, C. R., Lee, H., Goodarzi, H., Halberg, N. & Tavazoie, S. F. North 6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485 (2015).
-
Wang, 10. et al. N vi-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
-
Dominissini, D. et al. Topology of the human being and mouse m6A RNA methylomes revealed past 10006A-seq. Nature 485, 201–206 (2012).
-
Meyer, Chiliad. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and nigh finish codons. Jail cell 149, 1635–1646 (2012).
-
Harper, J. E., Miceli, S. M., Roberts, R. J. & Manley, J. 50. Sequence specificity of the man mRNA North 6-adenosine methylase in vitro. Nucleic Acids Res eighteen, 5735–5741 (1990).
-
Meyer, K. D. & Jaffrey, Due south. R. Rethinking mhalf dozenA readers, writers, and erasers. Annu Rev Prison cell Dev Biol 33, 319–342 (2017).
-
Liu, J. et al. A METTL3-METTL14 circuitous mediates mammalian nuclear RNA N six-adenosine methylation. Nat Chem Biol 10, 93–95 (2014).
-
Pendleton, K. E. et al. The U6 snRNA msixA methyltransferase METTL16 regulates SAM synthetase intron memory. Cell 169, 824–835 e814 (2017).
-
Warda, A. S. et al. Human METTL16 is a N six-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep xviii, 2004–2014 (2017).
-
Shima, H. et al. S-Adenosylmethionine synthesis is regulated by selective Northward 6-adenosine methylation and mRNA deposition involving METTL16 and YTHDC1. Jail cell Rep 21, 3354–3363 (2017).
-
Dark-brown, J. A., Kinzig, C. Thou., DeGregorio, Due south. J. & Steitz, J. A. Methyltransferase-like protein sixteen binds the 3′-concluding triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci United states of america 113, 14013–14018 (2016).
-
Sergiev, P. 5., Serebryakova, M. V., Bogdanov, A. A. & Dontsova, O. A. The ybiN gene of Escherichia coli encodes adenine-N half dozen methyltransferase specific for modification of A1618 of 23 South ribosomal RNA, a methylated residue located close to the ribosomal get out tunnel. J Mol Biol 375, 291–300 (2008).
-
Dorsett, One thousand., Westlund, B. & Schedl, T. METT-10, a putative methyltransferase, inhibits germ prison cell proliferative fate in Caenorhabditis elegans. Genetics 183, 233–247 (2009).
-
Kim, J., Kim, Y., Yeom, M., Kim, J. H. & Nam, H. G. FIONA1 is essential for regulating flow length in the Arabidopsis circadian clock. Plant Cell 20, 307–319 (2008).
-
Kozbial, P. Z. & Mushegian, A. R. Natural history of Due south-adenosylmethionine-bounden proteins. BMC Struct Biol v, xix (2005).
-
Martin, J. 50. & McMillan, F. G. SAM (dependent) I AM: the Due south-adenosylmethionine-dependent methyltransferase fold. Curr Opin Struct Biol 12, 783–793 (2002).
-
Schapira, Thousand. Structural chemistry of homo RNA methyltransferases. ACS Chem Biol 11, 575–582 (2016).
-
Schubert, H. 50., Blumenthal, R. Grand. & Cheng, Ten. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci 28, 329–335 (2003).
-
Wang, 10. et al. Structural basis of N 6-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578 (2016).
-
Wang, P., Doxtader, 1000. A. & Nam, Y. Structural ground for cooperative function of METTL3 and METTL14 methyltransferases. Mol Cell 63, 306–317 (2016).
-
Sledz, P. & Jinek, M. Structural insights into the molecular mechanism of the grand6A writer complex. Elife 5, e18434 (2016).
-
Iwaoka, M. & Isozumi, N. Hypervalent nonbonded interactions of a divalent sulfur atom. Implications in poly peptide architecture and the functions. Molecules 17, 7266–7283 (2012).
-
Malone, T., Blumenthal, R. Thou. & Cheng, X. Structure-guided analysis reveals nine sequence motifs conserved among Dna amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol 253, 618–632 (1995).
-
Wu, H., et al The crystal structure of human methyltransferase 10 domain containing protein. RCSB Protein Information Bank, PDB ID: 2h00 (2006).
-
Gong, W., O'Gara, G., Blumenthal, R. Chiliad. & Cheng, X. Construction of pvu II DNA-(cytosine N 4) methyltransferase, an example of domain permutation and protein fold assignment. Nucleic Acids Res 25, 2702–2715 (1997).
-
Schluckebier, One thousand., Zhong, P., Stewart, K. D., Kavanaugh, T. J. & Abad-Zapatero, C. The 2.two A structure of the rRNA methyltransferase ErmC' and its complexes with cofactor and cofactor analogs: implications for the reaction mechanism. J Mol Biol 289, 277–291 (1999).
-
Bugl, H. et al. RNA methylation under heat stupor control. Mol Prison cell 6, 349–360 (2000).
-
Martin, J. L., Begun, J., McLeish, M. J., Caine, J. M. & Grunewald, M. L. Getting the adrenaline going: crystal construction of the adrenaline-synthesizing enzyme PNMT. Construction 9, 977–985 (2001).
-
Komoto, J. et al. Crystal structure of guanidinoacetate methyltransferase from rat liver: a model structure of protein arginine methyltransferase. J Mol Biol 320, 223–235 (2002).
-
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res 32, W665–667 (2004).
-
Klimasauskas, S., Kumar, Due south., Roberts, R. J. & Cheng, X. HhaI methyltransferase flips its target base out of the DNA helix. Cell 76, 357–369 (1994).
-
Parker, B. J. et al. New families of human regulatory RNA structures identified past comparative assay of vertebrate genomes. Genome Res 21, 1929–1943 (2011).
-
Epstein, P., Reddy, R., Henning, D. & Busch, H. The nucleotide sequence of nuclear U6 (4.vii S) RNA. J Biol Chem 255, 8901–8906 (1980).
-
Bheemanaik, S., Reddy, Y. V. & Rao, D. Northward. Construction, part and machinery of exocyclic Deoxyribonucleic acid methyltransferases. Biochem J 399, 177–190 (2006).
-
Iyer, L. M., Zhang, D. & Aravind, 50. Adenine methylation in eukaryotes: Apprehending the complex evolutionary history and functional potential of an epigenetic modification. Bioessays 38, 27–40 (2016).
-
Gupta, Y. Thou., Chan, South. H., Xu, Southward. Y. & Aggarwal, A. K. Structural basis of asymmetric Dna methylation and ATP-triggered long-range diffusion by EcoP15I. Nat Commun half dozen, 7363 (2015).
-
Dorsett, M. & Schedl, T. A role for dynein in the inhibition of germ prison cell proliferative fate. Mol Cell Biol 29, 6128–6139 (2009).
-
Necci, Thousand., Piovesan, D., Dosztanyi, Z. & Tosatto, S. C. E. MobiDB-light: fast and highly specific consensus prediction of intrinsic disorder in proteins. Bioinformatics 33, 1402–1404 (2017).
-
Kim, I., McKenna, S. A., Viani Puglisi, E. & Puglisi, J. D. Rapid purification of RNAs using fast performance liquid chromatography (FPLC). RNA 13, 289–294 (2007).
-
Ke, S. et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are non required for splicing merely do specify cytoplasmic turnover. Genes Dev 31, 990–1006 (2017).
-
Linder, B. et al. Unmarried-nucleotide-resolution mapping of grandviA and m6Am throughout the transcriptome. Nat Methods 12, 767–772 (2015).
-
Ji, P. et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early on-stage non-small prison cell lung cancer. Oncogene 22, 8031–8041 (2003).
-
Piekna-Przybylska, D., Decatur, W. A. & Fournier, M. J. The 3D rRNA modification maps database: with interactive tools for ribosome analysis. Nucleic Acids Res 36, D178–183 (2008).
-
Liu, N. et al. Probing Northward 6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19, 1848–1856 (2013).
-
Sergiev, P. V., Aleksashin, N. A., Chugunova, A. A., Polikanov, Y. South. & Dontsova, O. A. Structural and evolutionary insights into ribosomal RNA methylation. Nat Chem Biol xiv, 226–235 (2018).
-
Kim, Y. et al. High-throughput protein purification and quality cess for crystallization. Methods 55, 12–28 (2011).
-
Kabsch, W. Xds Acta Cryst. D 66, 125–132 (2010).
-
McCoy, A. J. et al. Phaser crystallographic software. J Appl Crystallogr forty, 658–674 (2007).
-
Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. Automated macromolecular model building for Ten-ray crystallography using ARP/wARP version 7. Nat Protoc 3, 1171–1179 (2008).
-
Kowiel, Thousand., Jaskolski, M. & Dauter, Z. ACHESYM: an algorithm and server for standardized placement of macromolecular models in the unit jail cell. Acta Cryst. D 70, 3290–3298 (2014).
-
Emsley, P., Lohkamp, B., Scott, Westward. M. & Cowtan, 1000. Features and evolution of Coot. Acta Cryst. D 66, 486–501 (2010).
-
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst. D 67, 355–367 (2011).
-
Winn, M. D., Murshudov, 1000. N. & Papiz, 1000. Z. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol 374, 300–321 (2003).
-
Winn, M. D., Isupov, 1000. N. & Murshudov, G. N. Utilise of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Cryst. D 57, 122–133 (2001).
-
Painter, J. & Merritt, E. A. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Cryst. D 62, 439–450 (2006).
-
Brown, J. A., Valenstein, K. L., Yario, T. A., Tycowski, K. T. & Steitz, J. A. Formation of triple-helical structures past the three′-finish sequences of MALAT1 and MENβ noncoding RNAs. Proc Natl Acad Sci USA 109, 19202–19207 (2012).
-
Fischetti, R. et al. The BioCAT undulator beamline 18ID: a facility for biological non-crystalline diffraction and X-ray absorption spectroscopy at the Advanced Photon Source. J Synchrotron Radiat xi, 399–405 (2004).
-
Petoukhov, M. V. et al. New developments in the ATSAS program bundle for pocket-sized-bending scattering data analysis. J Appl Crystallogr 45, 342–350 (2012).
-
Konarev, P. Five., Volkov, V. V., Sokolova, A. Five., Koch, M. H. J. & Svergun, D. I. PRIMUS: a Windows PC-based system for small-bending scattering data analysis. J Appl Crystallogr 36, 1277–1282 (2003).
-
Svergun, D. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Crystallogr 25, 495–503 (1992).
-
Franke, D. & Svergun, D. I. DAMMIF, a program for rapid ab initio shape decision in modest-angle handful. J Appl Crystallogr 42, 342–346 (2009).
-
Volkov, V. V. & Svergun, D. I. Uniqueness of ab initio shape determination in small-angle scattering. J Appl Crystallogr 36, 860–864 (2003).
-
Svergun, D. I. Restoring low resolution structure of biological macromolecules from solution scattering using fake annealing. Biophysical Journal 76, 2879–2886 (1999).
-
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004).
-
Larkin, M. A. et al. Clustal Westward and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
-
Ashkenazy, H., Erez, East., Martz, E., Pupko, T. & Ben-Tal, N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38, W529–533 (2010).
-
Diederichs, K. & Karplus, P. A. Improved R-factors for diffraction data assay in macromolecular crystallography. Nat Struct Biol 4, 269–275 (1997).
Acknowledgements
This project was supported past NIH Grant R00GM111430, the Clare Boothe Luce Program of the Henry Luce Foundation, startup funds from the Academy of Notre Dame, and partially by the Intramural Research Plan of the NCI Center for Cancer Research. Diffraction data were collected at the SER-True cat beamline 22-ID at the Advanced Photon Source, Argonne National Laboratory, supported by the U.South. Department of Energy, Office of Scientific discipline, Office of Bones Energy Sciences under Contract Westward-31–109-Eng-38. This inquiry used resources of the Advanced Photon Source, a U.Due south. Section of Energy (DOE) Part of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. This project was supported by grant nine P41 GM103622 from the NIGMS. Use of the Pilatus 3 1 M detector was provided by grant 1S10OD018090-01 from NIGMS. The authors are thankful to Srinivas Chakravarthy (Biophysics Collaborative Access Team, Argonne National Laboratory) for his help during SAXS data drove and analysis, Patricia Clark (University of Notre Dame) for utilise of sonicator and Phillip McCown (Academy of Notre Dame) for a protein sequence alignment file of METTL16 homologs.
Writer information
Affiliations
Contributions
A.R. and J.A.B. designed experiments; A.R. performed the experiments; A.R., Thou.R., Z.D., and J.A.B. analyzed the results; A.R., M.R., and J.A.B. wrote the manuscript.
Respective author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional data
Publisher'south note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary textile
Rights and permissions
Open Admission This article is licensed nether a Artistic Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you requite appropriate credit to the original writer(south) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other tertiary party cloth in this article are included in the article'south Artistic Eatables license, unless indicated otherwise in a credit line to the textile. If material is not included in the article's Artistic Eatables license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you volition need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this commodity
Ruszkowska, A., Ruszkowski, M., Dauter, Z. et al. Structural insights into the RNA methyltransferase domain of METTL16. Sci Rep 8, 5311 (2018). https://doi.org/x.1038/s41598-018-23608-8
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-018-23608-eight
Further reading
Comments
By submitting a annotate you lot hold to abide past our Terms and Community Guidelines. If you find something calumniating or that does not comply with our terms or guidelines please flag it as inappropriate.
satterdonammis1999.blogspot.com
Source: https://www.nature.com/articles/s41598-018-23608-8/
0 Response to "U6 2d Motion - Review V31 Pg 4-8"
Enregistrer un commentaire